A jump in shingles after first Shingrix dose but the risk is transient, study finds
Key Takeaways
- Shingrix increases shingles risk 11-fold after the first dose, but risk decreases by 73% after the second dose.
- Temporary immune system changes may allow varicella zoster virus reactivation, causing shingles post-vaccination.
Australian researchers say the first shot may temporarily damp down the immune system, giving the latent varicella zoster virus a chance to activate and cause mild cases of shingles.
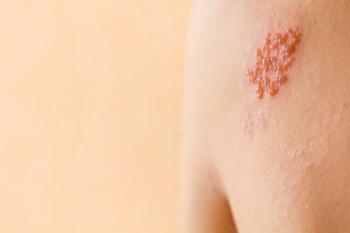
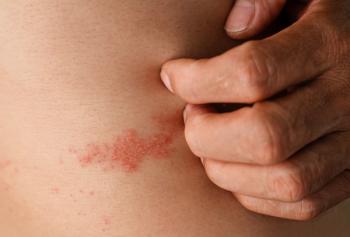
The risk of shingles jumps 11-fold after the first dose of the recombinant shingles vaccine, marketed under the name name Shingrix, but decreases by 73% after the second, according to the results of a study conducted in Australia.
First author
The shingles cases in the 21-day period between the first and second doses of Shingrix were mild, with no increased rate of antiviral prescribing or hospitalizations, the researchers noted. Once the second shot was given, the vaccine recipients had 73% reduction in shingles.
The study showed no increase of shingles in younger adults and no increase in postherpetic neuralgia.
Shingrix replaced Zostavax, a single dose vaccine that used a live attenuated varicella zoster virus, in Australia as it did in the U.S. When the switch was made in 2023, there was a flurry of reports from physicians and individuals of cases of shingles following the Shingrix shot. Those reports prompted Shetty and her colleagues to study the phenomenon in a more systematic way. They reported their findings in the journal Clinical Infectious Diseases, which published them online in September 2025 and then in the
The analysis by Shetty and her colleagues too in the period from Jan. 1,2023, through April 30,2025. They analyzed data from two datasets to explore the Shingrix-shingles issue, one that collects data from general practices in Australia and another on vaccine safety that is linked to hospital records. They sifted out shingles cases from the general practice data using Systematized Nomenclature of Medicine-Clinical Terms-coded diagnoses of herpes zoster. For the hospital data, they used an Australian version of the International Classification of Diseases, Tenth Revision.
Using the general practice dataset and the self-controlled case series method, an epidemiological design in which individuals act as their own control and commonly used to study vaccines, they found that the relative incidence of shingles in the 21 days after the first Shingrix shot was 10.96 times higher than it was during the prevaccination period in the overall study population and 12.20 times higher in the people 65 and older. In the dataset of hospital data, there was no increase in shingles in the overall population. In the older population, there was a suggestion of an increase, but it was not statistically significant.
The differences between the general practice and hospital data, they said, were not because shingles was being misdiagnosed by general practitioners. In fact, according to Shetty and her colleagues, the existing evidence shows that general practitioners’ herpes zoster diagnoses tend to be accurate. Rather, they wrote, the general practice-hospital difference was a reflection of the mildness of the shingles that occurs in the period after the first shot.
Despite the big jump in relative incidence, the results reported by Shetty and her colleagues showed that cases of shingles are relatively rare. In the older group of those 65 and older, they found that Shingrix was associated with 6.3 shingles per 1,000 first doses in the 21-day period after the first dose.
The researchers noted that the shingles rate decreased relative to prevaccination rates after the second shot of Shingrix. “This suggests that the risk is transient, brief, and likely mild and is elevated only in the short 21 days postvaccination,” they wrote.
Shingrix was approved by the FDA in October 2017 and became the only shingles vaccine available in the U.S. once Zostavax was discontinued in November 2020. The Center for Disease Control and Prevention (CDC) currently recommends that adults 50 and older get two doses of Shingrix, two to six months apart, to prevent shingles and related complications.
Shingrix replaced Zostavax for a number of reasons. Zostavax, a Merck product, was less effective than Shingrix. Moreover, its protective effects faded with time. It was also less effective in older people than Shingrix, and shingles disproportionately affects people who are older.
Newsletter
Get the latest industry news, event updates, and more from Managed healthcare Executive.