
Sanofi and GSK today announce will try to get approval from the FDA and the European Medicines Agency (EMA) for their COVID-19 vaccine, entering a crowded market.

Sanofi and GSK today announce will try to get approval from the FDA and the European Medicines Agency (EMA) for their COVID-19 vaccine, entering a crowded market.
With the COVID-19 pandemic ongoing and alarming levels of healthcare workers still experiencing stress, burnout, and other negative feelings surrounding their work, it's time to return the favors they've given countless others.

During the state of the industry webinar, AHIP experts reviewed policies and solutions to improve affordable access to care and shape healthcare in the post-pandemic world.

EmsanaRx, a pharmacy benefit manager launched by the Purchaser Business Group on Health, started operations in January.
The FDA’s authorization would depend on ongoing studies establishing that a fourth dose would shore up people’s molecular defenses that waned after their first booster shot.
New report includes research findings on insulin biosimilars use among retail pharmacists and physicians.

The CDC released the first real world data February 15 in a Morbidity and Mortality Weekly Report (MMWR) that infants younger than 6 months were overall 61% less likely to be hospitalized from COVID-19 if their mothers were vaccinated during pregnancy through completion of a 2-dose primary mRNA COVID-19 vaccine series with either Pfizer-BioNTech (Comirnaty) or Moderna (Spikevax).
A look at the challenges hospitals face due to an insufficient supply chain—and how to address them.

A vast majority said they plan to continue using telemedicine even beyond the pandemic, according to a report from Doximity.

There have been an alarming increase of ransomware attacks on healthcare systems in 2021—with more than 65 reported ransomware attacks on healthcare organizations in the third quarter alone and two-thirds of organization reporting that they had been targeted by ransomware strikes—a trend that is likely to continue in 2022.

The healthcare affordability crisis is having a detrimental impact on overall financial wellbeing and behaviors. In a recent Centivo survey, nearly three in five (59%) respondents say they had to make financial sacrifices due to significant medical expenses over the past two years.

Asundexian is an oral factor XIa inhibitor currently under phase 2 trials for potential secondary thrombosis prevention in patients with non-cardioembolic ischemic stroke, atrial fibrillation, or recent myocardial infarction.

The Department of Health and Human Services, through the Health Resources and Services Administration, awarded nearly $55 million to 29 HRSA-funded health centers to increase healthcare access and quality for underserved populations through virtual care such as telehealth, remote patient monitoring, digital patient tools, and health information technology platforms.
The solution, available immediately to all large health plans, is meant to simplify care for patients with complex conditions.

Last year was a record setting year in worldwide merger and acquisition activity across multiple industries, and healthcare was a big part of that.
An analysis cites increases in bloodstream and urinary tract infections. Hospitals and skilled nursing facilities are seeing more problems.

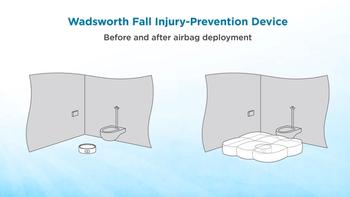
Main Line Health in suburban Philadelphia has solicited ideas for inventions from nurses. Barbara Wadsworth, the healthcare system’s chief operating officer with 35 years experience as a nurse, has invented a device for cushioning patient falls in the bathroom.

Hiring assistants that work virtually and outsourcing medical coding are two ways that providers can navigate through these times of increasing cost pressures and staffing shortages.

Adoption of digital health technologies came fast and furious in the pandemic, but now it’s up to insurers and patients to figure out the best recipe for a mix of remote and in-person care.

The FDA is postponing its Vaccines and Related Biological Products Advisory Committee meeting originally scheduled for February 15. Pfizer notified the agency about new data from its ongoing clinical trial.

In the sixth year of Medicare’s Independence at Home Demonstration program, the initiative saved $41 per member per month, an amount that CMS says was not statistically significant and was lower than the savings the program produced in earlier years.

Under the proposed rule, CMS would cover Aduhelm and other monoclonal antibodies for Alzheimer’s disease patients only if they are participating in approved clinical trials.

The Community Aging in Place—Advancing Better Living for Elders (CAPABLE) program developed at the university’s nursing school is designed to keep older people who are frail, have chronic medical conditions or can’t complete activities of daily living in their homes by providing care and help from conventional healthcare professionals — nurses, occupational therapists — but also from people who can do minor house repairs.

Keeping older people in their homes has cost and care advantages. Yet Medicare and Medicaid are still geared toward paying for care in institutionalized settings.
Medicare and Medicaid programs that serve the most-vulnerable Americans facing SDOH barriers can be major facilitators of appropriate non-emergency transportation to non-medical sites. But how do we determine what is appropriate, and what do we know about transportation services to non-medical sites today?

The questionnaire is seen as a low-risk, high-reward method of assessing asthma comorbidities in children.

Briana Contreras, editor of Managed Healthcare Executive spoke with Luis Mosquera, CEO of Health Network One, a provider of specialty benefit management services for health insurers, in this week's episode. In this discussion, Luis and Briana talked about how value-driven decisions in a more value-based market can not only better manage costs for health plans, but create a plan of care to best meet patient’s needs. In order to do this, Luis strongly encouraged healthcare executives to experience alternative payment models and shared what payers should check off their list with a new model.
