
The FDA is expected to make a decision on approval of the combination of Polivy with Rituxan and the R-CHP regimen by April 2, 2023.

The FDA is expected to make a decision on approval of the combination of Polivy with Rituxan and the R-CHP regimen by April 2, 2023.
Zoryve is a next-generation topical PDE4 inhibitor to treat adults and adolescents with plaque psoriasis.

If authorized, Novavax’s vaccine would be the first protein-based COVID-19 booster for adults.
Genentech has updated the warnings section of the label for the MS therapy Ocrevus to include information about cases of immune-mediated colitis and a serious brain infection in the post-marketing setting.
Those who spoke at a recent webinar discussed the prescription drug elements of the Inflation Reduction Act, which recently passed in the House, and the impact it will have on Medicare and its beneficiaries.

The four drugs recently launched include a generic of Clindagel, which received a competitive generic therapy designation from the FDA, for acne and Vasostrict for low blood pressure.

Investigation into additional agents to treat patients with COVID-19 through the I-SPY COVID Trial is ongoing.

Xofluza is a single-dose, oral therapy and is now approved for children five years and older for both the treatment and prevention of influenza.
Enhertu is the first HER2 directed drug to be approved for the treatment of patients with HER2 mutated metastatic non-small cell lung cancer.
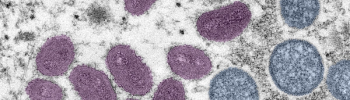
The EUA allows Jynneos to be used in children at high risk for monkeypox and as an intradermal injection, rather than subcutaneous, for adults.
The Vaccines and Related Biological Products Advisory Committee will hold a meeting on Sept. 22, 2022, to discuss Ferring’s RBX2660, a microbiota-based live biotherapeutic.

If approved, elacestrant would be the first oral selective estrogen receptor degrader (SERD) as a second- or third-line treatment for patients with ER+/HER2- advanced or metastatic breast cancer. The PDUFA date is Feb. 17, 2023.

Novaliq’s CyclASol uses a new technology that allows cyclosporine to be soluble without water or preservatives.

A rise in the number of long COVID is sparking research efforts by the HHS, the CDC and drug companies to understand the conditions and develop treatments.

The updated PDUFA date is Feb. 28, 2023, and the planned advisory committee meeting is on hold pending review of NDA amendments.

The approval had been delayed as the regulatory agency reviewed data on Myfembree's impact on bone mineral density.

Susan Lang, CEO and founder of XIL Health, talks about the shifting trends and pressures that have led to a misalignment between the PBMs, their clients and consumers.
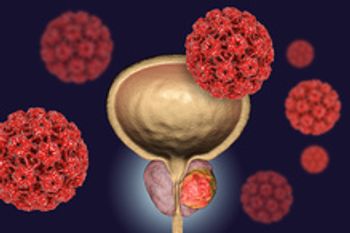
Abiraterone is used together with prednisone to treat patients with metastatic prostate cancer.

Confirmed cases of monkeypox in the United States have reached more than 7,500, according to the CDC.

Nubeqa, in combination with docetaxel, is now approved to treat metastatic prostate cancer.
The Inflation Reduction Act also allows Medicare to negotiate prices on some drugs and caps out-of-pocket costs for Medicare beneficiaries.

The tablet form has the same safety and efficacy as the capsule formulation and allows it to be administered with gastric-reducing therapies.

This is the first approved therapy targeted to patients with the HER2-low breast cancer subtype. Its approval comes four months ahead of its PDUFA action date.

The committee will meet on Sept. 7, 2022, to discuss additional analyses of data from the Amylyx’s clinical studies. The updated PDUFA goal date is Sept. 29, 2022.
The FDA has requested Acadia conduct an additional trial of Nuplazid to support an indication in patients with Alzheimer’s disease.

Fresenius Kabi expects to receive FDA approval for its tocilizumab biosimilar in 2023 and is seeking approval in both intravenous and subcutaneous administration.