Several companies are developing new drugs to treat transthyretin amyloidosis cardiomyopathy (ATTR-CM), a formerly obscure condition that is getting an increasing amount of attention.

Several companies are developing new drugs to treat transthyretin amyloidosis cardiomyopathy (ATTR-CM), a formerly obscure condition that is getting an increasing amount of attention.

How health plans are implementing strategies to leverage biosimilars to lower costs for both payers and patients, according to Javier Gonzalez, Pharm.D., chief growth and commercial officer of Abarca

Cynthiya Ruban, Ph.D., M.S., Director of Digital Solutions at Cencora says to keep the following in mind as AI use accelerates.

Ways AI is increasingly used in healthcare and how it can benefit healthcare decision makers, according to Cynthiya Ruban, Ph.D., M.S., Director of Digital Solutions at Cencora.

It's not the multimillion dollar prices, says Kevin Niehoff, Pharm.D., of IPD Analytics. The pool of sickle cell disease patients who are prime candidates for sickle cell disease gene therapy was overestimated, according to Niehoff.

Only a small group of patients have started the process to receive the new gene therapies for sickle cell disease, says Kevin Niehoff, Pharm.D., of IPD Analytics.

Just a small percentage of employers use alternative funding programs, including accumulators and maximizers. But approximately 60% of employers who do use these programs said in a survey they are effective at managing healthcare costs.
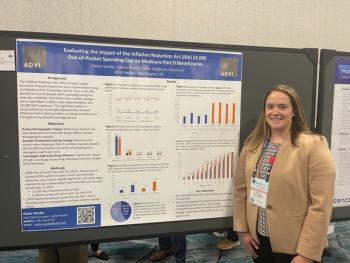
Prior to the IRA, there were no limits on beneficiary OOP spending throughout the year. However, by 2025 the IRA will introduce the $2,000 maximum OOP expenses.

The Minnesota-based pharmacy benefit manager says a program that resulted in patients switching from two incretin therapy prescriptions to one yielded $7,500 in savings per patient and a total of $3.5 million.

In a platinum award winning poster presented at the AMCP Nexus 2023 conference in Orlando, “Specialty drug use varies by race and wage among employees with employer-sponsored health insurance,” authors expressed that spending on specialty medications for autoimmune conditions has increased in recent years, raising affordability concerns for employers.
Research continues to develop new therapies for rare cancers and to provide options that allow for fewer toxicities. At the same, however, more new drugs are launching with high price tags.

Adopting evolving computer system tools like artificial intelligence and machine learning in managed care pharmacies have resulted in efficiency when addressing the challenges they are faced with, according to Jessica Hatton, PharmD, BCACP, associate vice president of Pharmacy at CareSource and Nick Trego, PharmD, senior vice president of Clinical Analytics and Client Services at HealthPlan Data Solutions, Inc.

The program yielded savings of $25,000 per patient in its pilot phase but is not expected to produce savings as a routine offering because reimbursement for home infusion was matched to reimbursement at a facility. Horizon executive Timothy O’Shea, Pharm.D., M.S., says cost savings were a “secondary outcome” of the program and noted the high patient satisfaction.

Just over 160 patients have participated in the insurer's oncology home infusion program since it started in late 2020. Patient satisfaction is high, according to Horizon officials, who are looking to expand the program with other providers in its market and to include more drugs that patients could be treated with at home.

Using an AI platform, Magellan Health was able to better support providers in prescribing behavioral health medications and addressing medication problems, which reduced pharmacy costs and increased adherence.

Jessica Hatton, PharmD, BCACP, associate vice president of Pharmacy at CareSource caught up with MHE to discuss value assessment tools and their use in the managed care pharmacy space. This topic and more were addressed by Hatton during her presentation today in Orlando at the AMCP Nexus 2023 conference.

The results of a recent feasibility study on the outpatient administration of cell therapies is creating growing interest in whether home-based management may be possible in the future.

In a session presented at the AMCP Nexus 2023 conference in Orlando, Adam Colborn, JD, director of Government Relations at AMCP, discussed how the recent ERISA preemption rule influenced employer pharmacy benefits, and also highlighted a policy that hasn't received much attention in the managed care pharmacy space: California's CalRx Biosimilar Insulin Initiative.

Adam Colborn, JD, director of Government Relations at AMCP, addressed topics at this year's AMCP Nexus conference in Orlando such as transparency, cost sharing mandates and variables that affect the supply and demand curve for pharmaceuticals within legislation at a federal, but mainly state level. Colborn also touched on the large PBM reform regulation happening currently in New York as it could be a model that other states can follow.

In her review of the specialty drug pipeline, Evernorth's Aimee Tharaldson said upcoming approvals for Crohn’s and colitis drugs could further shift the drug spend from the medical benefit to the pharmacy one.

Patient affordability and access to specialty drugs and cell and gene therapies are just a couple of priorities self-insured employers should keep in mind when managing specialty spend, according to Shawna Ricker, PharmD, clinical pharmacist consultant of Clinical Client Strategy at Highmark, Inc.

Shawna Ricker, PharmD, Clinical Pharmacist Consultant, Clinical Client Strategy, Highmark, Inc., who co-presented at this year's AMCP Nexus conference in Orlando, shares some best practices for health plans that work with self-insured employers.
A pharmacist-led program to switch patients to biosimilars at the Mayo Clinic improved access and lowered costs, the clinic's Chelsee Jensen, Pharm.D., said during a presentation today.

Melissa Andel, M.P.P., of CommonHealth Solutions LLC, says there has been some retreat from earlier proposals for severe regulation to an emphasis on disclosure and transparency.
Melissa Andel, M.P.P., of CommonHealth Solutions LLC, thumbnailed the four pieces of legislation advancing through Congress that would tighten regulation and oversight of pharmacy benefit managers (PBMs).

Attendance rebounded to pre-pandemic levels at the AMCP Nexus 2022 meeting in National Harbor, Maryland, and very few attendees worse masks.

Chester "Bernie" Good, senior medical director for the Center for Value Based Pharmacy Initiatives at UPMC Health Plan, gets in depth on value-based contracts and shares the results of the 2019 contract between UPMC and AstraZeneca for Brilinta. Good addressed value-based contracts last week at AMCP Nexus 2022 conference in National Harbor, Maryland.
EQRx has said it will price its PD-1/PDL-1 inhibitor 40% below the price of the entrenched PD-1/PDL-1 inhibitors, says Bhavesh Shah of Boston Medical Center Health System. But there are obstacles looming, including the development of combination therapies.

Julie Kendle, Director, Clinical Pharmacy Director, Clinical Pharmacy at IPD Analytics addresses tips on improving accumulators and ways for PBMs or payers to make their accumulator and maximizer programs more acceptable to patients. Kendle spoke at this year's AMCP Nexus meeting in National Harbor, Maryland.
A real-world study by the PBM shows major benefits from the new cystic fibrosis drug when it comes to hospitalizations and pulmonary exacerbations. But total cost of care of patients tripled because the drug is expensive.