
After three months of recovery, financial and volume indicators were off in August, according to Kaufman Hall's monthly report.

After three months of recovery, financial and volume indicators were off in August, according to Kaufman Hall's monthly report.

CMS says 26.9 million beneficiaries are expected to enroll in Medicare Advantage plans in 2021.

American Society of Anesthesiologists opposes drastic payment reduction for critical services.
The American Medical Association and American Heart Association reaffirm commitment to working together to equip physicians and all Americans, particularly communities of color, with resources to lower blood pressure rates across the country.

A session at MSVirtual2020 covers the difficulty of finding treatments for some forms of MS.

Data presented on a pair of Novartis MS therapies during MSVirtual2020.

Trials taking place fall 2020, the study subset will focus on favipiravir’s potential to reduce viral shedding, diminishing the period of infectiousness.

According to a report by L.E.K Consulting, challenges are unlike other industries’, and adoption of artificial intelligence may be slow. Telemedicine accelerated by COVID-19 could drive uptake.
The New York Times is reporting that a study volunteer in the U.K. developed transverse myelitis.

One code covers additional supplies and clinical time related to safety protocols. The other covers antibody testing.
The letter, “Dentists Fighting Covid” states, in part: “We believe your recommendation is irresponsible, unsubstantiated and dead wrong."
Four initiatives healthcare operators can implement to help their employees deal with the challenges posed by the pandemic.

Pro, college, and high school athletes are seeing increasing numbers of COVID-19 infection and potential heart complications that could be career-ending. Ambulatory cardiac monitoring provider InfoBionic says simple and effective remote cardiac monitoring will save lives—and careers.

A JAMA Oncology viewpoint argues that the COVID-19 pandemic presents a prime opportunity to make clinical trial design and execution more efficient.

Findings presented at the European Society of Cardiology meeting could open the door to the use of the gout drug as a treatment targeting the underlying inflammation of cardiovascular disease.

This week's FDA updates include an end to a boxed warning for a type 2 diabetes drug, an expanded emergency use authorization due to COVID-19, and another liquid biopsy.

Integrated Benefits Institute study finds increased screenings for depression and detection of chronic conditions before symptoms present could have greatest impact on disability costs.

Scientists react negatively to FDA's handling of the Emergency Use Authorization for convalescent plasma in COVID-19.

FDA actions for the week ending August 21, 2020, include a hearing set for a drug that could limit weight gain often associated with treatment for serious mental illness.

Survey of Midwestern ophthalmologists identifies reasons for wariness of generics.
As the American population ages, chronic conditions are becoming an increasing challenge for payers. In addition to conditions such as cardiovascular disease and diabetes, eye disease is also prevalent among aging adults.
Almost four million people miss their medical appointments every year due to a lack of reliable transportation. To address this challenge, Lyft has been working with healthcare organizations since 2016 to get patients in need to and from their medical appointments.

There are several explanations for the growing number of nearsightedness people.Time spent looking at computer and cellphone screens has been identified as a culprit.

Expert William J. Mallon, M.D., discusses the most-common eye diseases seen in practice.


Authors of a review say biosimilars would improve treatment, not just reduce cost.
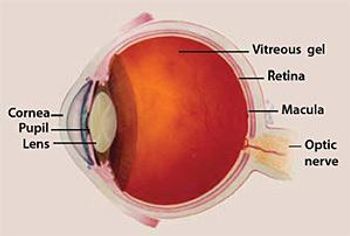
Real-world evidence from 67 patients recently found benefits for those with diabetic macular edema who switched to Eylea from Lucentis.
Whether the OCM has worked out is debatable, but it has apparently encouraged the use of biosimilar versions of filgrastim.