
FDA approved a new antibiotic to treat community-acquired bacterial pneumonia (CABP) and acute skin and skin structure infections (ABSSSI).

FDA approved a new antibiotic to treat community-acquired bacterial pneumonia (CABP) and acute skin and skin structure infections (ABSSSI).
A new study linking attention-deficit hyperactivity disorder (ADHD) drugs and Parkinson’s disease could cause prescribers to re-think prescribing the medications.


Here are the top 3 new approvals from FDA.

While Teva Pharmaceutical’s new migraine prevention drug is unique, it faces competition from another major migraine treatment.
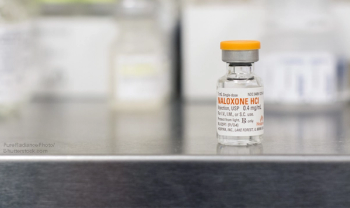
Pharmacy and healthcare experts cautiously praised the planned development of a fast-acting, better version of naloxone (Narcan), which is used to reverse the effects of opioid overdoses.

FDA okayed a first-of-its-kind treatment for hairy cell leukemia (HCL).

FDA recently approved 2 new treatments, heightening competition in the HIV-1 drug marketplace.

FDA cleared the first and only easy-to-swallow liquid drug to treat amyotrophic lateral sclerosis (ALS).

A pharma maker is recalling one lot of an allergy medication because the bottles mistakenly contain this medication.

FDA is warning that a deadly infection has been reported with a widely used class of diabetes drugs.

FDA recently approved a new treatment for 2 rare types of non-Hodgkin lymphomas, as well as a new drug to treat hereditary angiodema and 1 for Fabry disease. Here are the top three new drugs to treat rare diseases.

While FDA approved the first drug containing cannibidiol (CBD), it is cracking down on certain other CBD products marketed as supplements.

FDA clears first-of-its-kind gene therapy for rare disease.

While FDA approved the first generic version of epinephrine (EpiPen and EpiPen Jr, Mylan), the new product is not yet on the market.

Pharmaceutical industry observers are concerned about some of the drugs that were slashed from Express Scripts’ 2019 National Preferred Formulary.
The first medication approved by FDA as part of an expedited generic drug program is one that has been in short supply.

FDA okayed iobenguane I 131 (Azedra, Progenics Pharmaceuticals) injection for IV use to treat rare tumors of the adrenal gland.

FDA recently approved the first and only complete darunavir-based single-tablet regiment to treat HIV-1 in treatment-naïve and certain virologically suppressed adults.

A breast cancer drug is the first approval from FDA in 2 new pilot programs that make the development and review of cancer drugs more efficient.


FDA’s approval of an expanded indication for a prostate cancer drug could net 2 pharmaceutical manufacturers billions of dollars.

Pharma makers are voluntarily recalling several drugs containing this active ingredient, used to treat high blood pressure and heart failure, since they may contain a carcinogen.

FDA okayed the first treatment for chronic sialorrhea, or excessive drooling, in adult patients.

A pharma maker is under fire for raising prices on its cancer drugs. Meanwhile, there were two major cancer drug approvals in June. Here are the top 3 cancer news articles in the past month.