
Health groups urge Trump to appoint Acting FDA Commissioner Ned Sharpless.

The new drug is an add-on to levodopa/carbidopa for Parkinson’s patients experiencing “off” episodes.

Use of these meds to treat chronic hepatitis C in patients with moderate to severe liver impairment has resulted in liver failure in rare cases.
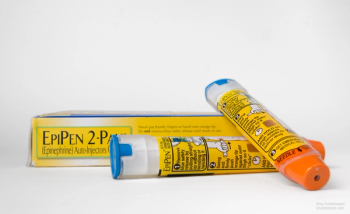
Teva’s generic EpiPen Jr. helps alleviate allergy drug shortage.

DEA scrambles to add qualified marijuana growers.

FDA approved the third oncology drug in the US that targets a key genetic driver of cancer, rather than a specific tumor.
Blockbuster drug could be key to Humira’s successor.

While FDA’s drug approval time is generally improving, the agency needs to speed up generic drug application reviews, according to a new report.
While FDA recently said Novartis and AveXis did not reveal data on their $2.1 million gene thaerpy for pediatric spinal muscular atrophy until after it was approved, Novartis is defending its actions.
Drug to treat prostate cancer is expected to produce a major windfall for two pharma makers.

FDA approved yet another indication for pembrolizumab (Keytruda) to treat patients with recurrent, locally advanced or metastatic, squamous cell carcinoma of the esophagus (ESCC).

FDA is adding an important new Boxed Warning on the higher-dose version of this drug.
FDA approved the first and only ready-to-use insulin for IV infusion in hospitals and acute care settings.

Patients with overactive bladder taking this OAB drug did not experience an excess risk of CV events compared with other treatments.
Asthma patients may soon treat themselves with first-ever digital maintenance inhaler.

While some are concerned that President Trump’s proposed “favored nations” policy would harm pharma makers, one analyst is not as concerned.

While some are concerned that President Trump’s proposed “favored nations” policy would harm pharma makers, one analyst is not as concerned.
EpiPens have been in short supply, but stakeholders are trying to fill in the access gap.
FDA warns opioid repackers for "significant violations."
FDA granted Fast Track designation to a popular diabetes drug to reduce the risk of cardiovascular death and hospitalization for heart failure in people with chronic heart failure.

The validity of a new large study linking anticholinergic drugs to the risk of dementia has stirred up controversy.
FDA recalls insulin pumps due to cybersecurity miscommunication.

A women’s health organization is concerned about the safety and effectiveness of bremelanotide (Vyleesi) after FDA approved it to treat hypoactive sexual desire disorder (HSDD) in premenopausal women.

A new report from OptumRx reveals the top 4 drugs in development for the second quarter of 2019.
The first FDA-approved device to reduce functional abdominal pain in younger patients with irritable bowel syndrome is now available to medical providers.
A record number of new oncology treatments launched in the US last year and spending on cancer drugs soared. Here are the top 6 cancer drug trends revealed in the report.