
The standard of care does not control chronic rhinosinusitis with nasal polyps for a third of patients, but approved biologics may improve quality of life (QoL).

The standard of care does not control chronic rhinosinusitis with nasal polyps for a third of patients, but approved biologics may improve quality of life (QoL).
A therapy that rapidly demonstrates improvements in clinical outcomes may have a positive impact on medication adherence.

Research presented at the 2022 American Academy of Allergy, Asthma & Immunology (AAAAI) Annual Meeting found minority patients with asthma perceived lower quality interactions with their care team, but did not find significant differences in the prescription of biologic medications across racial/ethnic groups.

The FDA issued a complete response letter, so AstraZeneca’s monoclonal antibody won’t be joining Nucala and Dupixent as treatments for chronic rhinosinusitis with nasal polyps anytime soon.

A meta-analysis finds that providing both parents and children with asthma with information about managing their disease can reduce emergency room visits and hospitalizations.
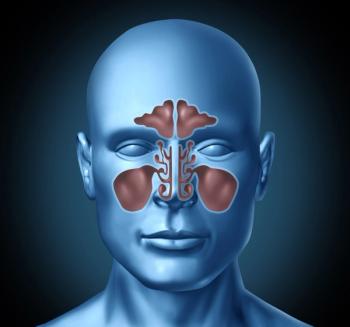
A recently published review delves into the role that type 2 inflammation plays chronic rhinosinusitis and the evidence that agents that interfere with that inflammatory pathway could be used as treatments.
Safety concerns may limit JAK inhibitors as treatment for the common skin condition.

Rinvoq, a JAK inhibitor, is still under review at the FDA as a treatment of atopic dermatitis.
Incyte will be required to conduct postmarketing study and set up patient registry to monitor whether the topical JAK inhibitor has adverse effects on mothers, fetuses and infants.