
The combination of Spinraza and Zolgensma could improve clinical outcomes in infants with spinal muscular atrophy without added risks.

The combination of Spinraza and Zolgensma could improve clinical outcomes in infants with spinal muscular atrophy without added risks.
A new study finds that that eczema can worsen when air quality drops from good to moderate levels on the Environmental Protection Agency’s Air Quality Index.
Preserving the ability to swallow reduces the need for invasive interventions and lowers the risk of life-threatening complications such as aspiration pneumonia.

Researchers reviewed 900 Reddit posts to assess opinions about the increasingly popular class of drugs.

Roche’s Evrysdi is the market leader among the drugs for spinal muscular atrophy. Biogen’s Spinraza has struggled, but company officials express confidence about future sales.
In a meta-analysis of 57 studies, Zolgensma achieved a 95% survival rate and reduced the number of patients requiring ventilatory support.

The campaign for hand eczema is the latest in a number of high-cost campaigns to raise awareness of dermatological conditions.
Repigmentation case is just the third that has been reported.

The study drew on data from the RESTORE registry for 34 patients with tracheostomies who received Zolgensma (onasemnogene abeparvovec).

Roche’s Evrysdi (risdiplam) is the market leader among the drugs for spinal muscular atrophy (SMA). Biogen’s Spinraza (nusinersen) has struggled, but company officials express confidence about future sales.

Ebglyss also provided meaningful improvements in difficult-to-treat face and hand dermatitis.

In a response to a survey, caregivers of people with spinal muscular atrophy identified the risk of severe adverse events and the need for permanent ventilation as the most important factors in treatment decisions. Access to treatment, including cost and availability, ranked third.

Some prior research had shown that gene therapy would not lower the amount of time children spend on ventilator support.
Research shows that after 60 months of treatment, 38% of patients achieved complete remission.

Two years after treatment, both girls show normal motor function and no signs of spinal muscular atrophy symptoms.

Mental health and vitiligo treatment outcomes may be associated because of a brain-skin axis, a pathway that links emotional stress to skin conditions.

Apitegromab works by targeting a protein called myostatin that limits muscle growth and is designed to be used alongside existing spinal muscular atrophy (SMA) treatments.

Previous research has shown an association between atopic dermatitis and obesity. But these findings elaborate on that observation and point to a connection to abnormal lipid metabolism and elevated "bad" LDL cholesterol levels.

Study of 29 children treated with Zolgensma (onasemnogene abeparvovec) shows mixed results.

This is the first study to investigate the effectiveness of this combination to treat vitiligo.
U.S. providers surveyed said that getting insurance approval is time-consuming and can delay treatment for newborns with spinal muscular atrophy.
Lebrikizumab, not yet approved in the U.S., and Dupixent (dupilumab) were comparably effective in reducing symptoms after 16 weeks of treatment.


Apitegromab, an investigational drug for spinal muscular atrophy, demonstrated sustained patient benefits and maintained safety over a three-year period in a phase II study, with 88% of participants maintaining or improving motor milestones.

The system, called ThecaFlex DRx, allows subcutaneous administration of Spinraza (nusinersen) directly into the cerebrospinal fluid, and aims to replace the current administrative route through lumbar punctures.


SMA, a rare genetic neurodegenerative disorder affecting approximately 1 in 10,000 newborns, is typically fatal for untreated children by age two. However, the approval of disease-modifying medications have transformed the treatment landscape.

Risdiplam is an oral small molecule treatment that impacts both the central nervous system and peripheral tissues. Patients receive the medicine daily in liquid form either by feeding tube or by mouth. The drug was first approved by the FDA in 2020 for patients as young as two-months old.
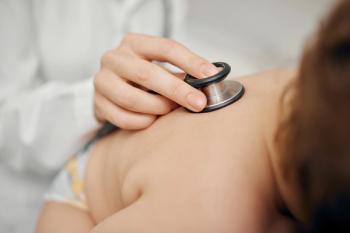
According to a study published in JAMA Pediatrics, screening newborns for spinal muscular atrophy (SMA) and starting treatment immediately can significantly improve health outcomes compared to infants who are diagnosed and begin treatment months later.