New Advertising Campaign Aims to Raise Awareness of Chronic Hand Eczema
The campaign for hand eczema is the latest in a number of high-cost campaigns to raise awareness of dermatological conditions.
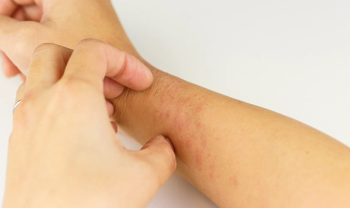
The latest skin disease to be spotlighted in a marketing campaign is chronic hand eczema (CHE), a debilitating condition affecting approximately 4.7% of adults. The advertising effort, by LEO Pharma, joins a crowded field of drugmakers attempting to raise awareness – and sales – for atopic dermatitis treatments.
Leo’s "Talk to the Hand" campaign,
“Our hands are a part of everything we do and who we are — It is essential for healthcare providers to understand that CHE is not just atopic dermatitis of the hands,” said Omar Noor, M.D., co-owner of Rao Dermatology, in the LEO news announcement.“We need to recognize the unique challenges of CHE so that we can more effectively diagnose and care for those across the U.S. living with this condition.”
Leo’s initiative follows other prominent advertising efforts in dermatology. Pfizer's
The top spenders in the space, however, are Sanofi and Regeneron. The companies marketed Dupixent (dupilumab), a biologic treatment for moderate to severe atopic dermatitis, with ad campaigns costing $523.9 million in 2021 and $491 million in 2022, the most of any drug during those years, according to
Although pharmaceutical companies lead in advertising spending, patient advocacy organizations also spend significantly to disease awareness efforts. The National Eczema Association (NEA), for instance, holds annual campaigns, including
"We're excited about LEO Pharma's "Talk to the Hand" campaign to help shed light on the unmet need for the diagnosis and care for patients living with chronic hand eczema," said Kristin Belleson, President and CEO of NEA, in the LEO news release.
LEO’s treatment for CHE is Anzupgo (delgocitinib), a topical pan-Janus kinase (JAK) inhibitor designed to treat adults who have not responded adequately to topical corticosteroids.. Anzupgo is
“When dealing with clinicians, we want to look at science and symptoms,” said Brian Hilberdink, Executive Vice President and President of Region North America at LEO Pharma, said during a
Newsletter
Get the latest industry news, event updates, and more from Managed healthcare Executive.