Case Study Highlights Risk of Syringe-related SODs Following Syfovre Injection
The patient was in her 70s and began experiencing the floaters the day after undergoing treatment.
A new case study discusses a patient who experienced small floaters in her eye after undergoing an intravitreal injection (IVI) of pegcetacoplan.
The findings were published this month in a research letter in JAMA Ophthalmology.
Jacques Bijon, M.D.

Previous studies have suggested a link between floaters following an IVI of anti–vascular endothelial growth factor agents and gas bubbles, vitreous hemorrhage, and syringe-related silicone oil droplets (SODs), explained first author Jacques Bijon, MD, of the New York University Grossman School of Medicine, and colleagues.
SODs are translucent bodies that appear in a sphere shape on hyperechogenic B-scan ultrasonography, they said. The droplets were first reported after patients received an IVI of pegaptanib sodium in 2006.
Pegcetacoplan was approved in 2023 to treat geography atrophy. According the American Academy of Ophthalmology, the condition that affects nearly one million Americans, and can inhibit individuals’ ability to recognize faces, read, and drive.
The patient in the case study was in her 70s, had no history of intraocular surgery or IVIs, but had bilateral center-involving geographic atrophy.
She presented with bilateral posterior vitreous detachments and had a single IVI of pegcetacoplan administered to her left eye. It was injected according to the treatment’s prescribing information.
Previous studies have suggested a link between floaters following an intravitreal injection of anti–vascular endothelial growth factor agents and gas bubbles, vitreous hemorrhage, and syringe-related silicone oil droplets.
Credit: weyo - stock.adobe.com
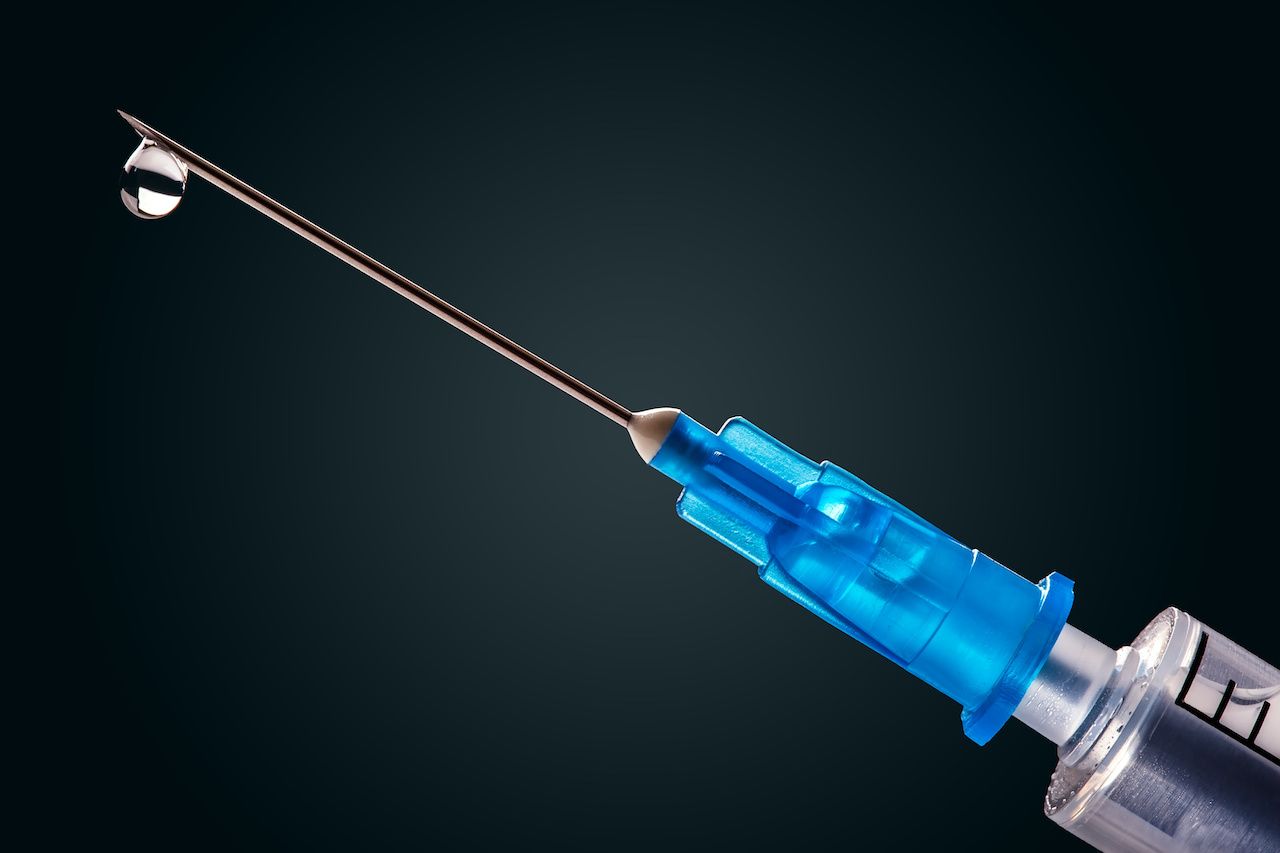
Two weeks after receiving the injection, she reported she began to experience multiple small floaters that began the day after the treatment.
At the two-week mark, evaluations revealed she had stable visual acuity and normal intraocular pressure. The patient had no anterior segment or vitreous inflammation, but the evaluation did show multiple SODs in the anterior vitreous of her left eye, Bijon and colleagues wrote.
A subsequent ultrasonography also revealed hyperechogenic areas within the detached vitreous in the affected eye, they said.
After two months, a follow-up was carried out. “The patient reported reduced floaters, but SODs remained visible in the anterior vitreous,” Bijon and colleagues said.
Although they were first reported in 2006, the American Society of Retina Specialists sent out an alert on reports of SODs linked with intravitreal bevacizumab treatments administered via siliconized insulin syringes in 2016.
A study carried out the following year compared different syringe types used for IVIs. In this study, researchers observed “SODs with fixed needle syringes appearing at the end of injections as the plunger was fully depressed,” explained Bijon and colleagues.
However, no droplets were seen when detachable needle syringes that have dead space at the tip were used, leading researchers to hypothesize this dead space could retain fluid that was noninjectable. This fluid could contain the majority of SODs identified at the end of injection, as it has higher viscosity and hydrophobicity than aqueous fluid, they said.
When prepping to administer pegcetacoplan, gas bubbles are formed from the dead space at the syringe’s tip and needle. These bubbles can then enter the syringe before the drug.
The label’s instructions read “Do not tap the syringe to remove air bubbles. While maintaining the filter needle within the vial, invert the syringe and move the plunger down and up until bubbles move to the top,” said Bijon and colleagues.
But these steps could lead to SODs entering patients’ eyes. To mitigate the risk of this occurring, authors suggest ophthalmologists utilize silicone-free Luer lock syringes.
Using a filtered vial adaptor— as researchers did in pegcetacoplan’s phase III studies—could also “simplify drug removal with the vial inverted so that gas bubbles may remain at the syringe tip where they can be more easily expelled before IVI,” Bijon and colleagues concluded.
Briana Contreras, editor of Managed Healthcare Executive, spoke with Nancy Lurker, CEO and president of EyePoint Pharmaceuticals. Nancy shared a bit about EyePoint and how the organization’s innovative therapies are addressing patient needs through eye care, and most importantly, she addressed C-Suite positions like the CEO role. Nancy shared advice for those seeking to reach the CEO level, especially toward women in healthcare and other roles, and what it takes to run a biopharma company.
Listen
Researchers Gain Insight into Eye Side Effects of Blenrep
May 20th 2025A case analysis provides a new understanding of the ocular side effects of Blenrep, an antibody drug conjugate that is being reviewed by the FDA as a combination treatment for patients with multiple myeloma. The goal date is July 23, 2025.
Read More
Briana Contreras, editor of Managed Healthcare Executive, spoke with Nancy Lurker, CEO and president of EyePoint Pharmaceuticals. Nancy shared a bit about EyePoint and how the organization’s innovative therapies are addressing patient needs through eye care, and most importantly, she addressed C-Suite positions like the CEO role. Nancy shared advice for those seeking to reach the CEO level, especially toward women in healthcare and other roles, and what it takes to run a biopharma company.
Listen
Researchers Gain Insight into Eye Side Effects of Blenrep
May 20th 2025A case analysis provides a new understanding of the ocular side effects of Blenrep, an antibody drug conjugate that is being reviewed by the FDA as a combination treatment for patients with multiple myeloma. The goal date is July 23, 2025.
Read More
2 Commerce Drive
Cranbury, NJ 08512
All rights reserved.