Anzupgo FDA Approved as First Treatment Meant for Chronic Hand Eczema in Adults
The FDA approved Anzupgo (delgocitinib) cream today as the first and only treatment specifically for adults with moderate-to-severe chronic hand eczema (CHE) in the United States.
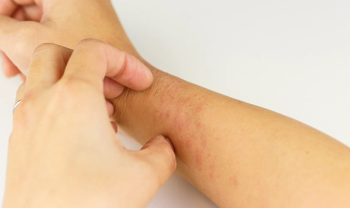
Chronic hand eczema is a long-lasting, often painful skin condition that affects approximately one in ten adults worldwide. It’s characterized by
Developed by LEO Pharma, Anzupgo is a steroid-free, topical pan-Janus kinase (JAK) inhibitor.
It works by blocking multiple components of the JAK-STAT signaling pathway—specifically JAK1, JAK2, JAK3 and the Tyrosine Kinase 2 (TYK2) proteins—which are involved in the inflammatory processes behind CHE. By directly targeting the source of inflammation, Anzupgo helps manage symptoms and prevent flare-ups in patients who haven’t responded to, or cannot use, corticosteroids.
Anzupgo cream is available in a 2% formulation, with each gram containing 20 mg of delgocitinib in a white to slightly brown cream. The FDA-approved
According to public pricing data, the
“Anzupgo is a good example of how we transform a real need in the market into medicines that can help make a difference for people living with serious skin diseases such as CHE,” Christophe Bourdon, CEO of LEO Pharma, said in
The approval is based on two pivotal phase 3 clinical trials—
In both trials, patients using Anzupgo cream achieved significantly higher rates of treatment success—defined as clear or almost clear skin—compared to those using the placebo cream. The study resulted in 20% of treated patients in DELTA 1 and 29% in DELTA 2 that met the primary endpoint, compared to just 10% and 7% in the placebo groups. The treatment was also well tolerated, with similar rates of side effects across all groups.
The decision also comes after prior regulatory approvals in Europe and the Middle East, including in Germany, the U.K., Switzerland and the United Arab Emirates (UAE). In these countries, Anzupgo has already been used to treat adults struggling with the daily burdens of CHE.
CHE is considered one of the most debilitating skin conditions due to its visibility and interference with daily life. Studies show that roughly 70% of those with severe CHE report difficulty performing everyday tasks such as cooking, working, or washing hands. The condition is also associated with significant psychological stress and, in many cases, negatively impacts a person’s ability to work, affecting long-term income and job retention.
“Chronic hand eczema can be a very difficult disease for adults to manage, especially given the lack of treatment options in the U.S. until now,” Robert Spurr, executive vice president and president of North America at LEO Pharma, said in the release.
The manufacturer recommends that Anzupgo not be used with other JAK inhibitors or strong immunosuppressants. It’s intended for adults who have experienced poor responses to corticosteroids or for those whose treatments are not suitable.
As the first treatment approved specifically for CHE in the U.S., Anzupgo marks a significant step forward in providing long-overdue relief for patients who have struggled without effective options.
Newsletter
Get the latest industry news, event updates, and more from Managed healthcare Executive.