
Clinical data suggests an imbalance in vaso-occlusive crises and fatal events that require further assessment, and the company said it will provide updates in the future.

Clinical data suggests an imbalance in vaso-occlusive crises and fatal events that require further assessment, and the company said it will provide updates in the future.
Veklury is indicated to treat adults and children who are hospitalized with COVID-19.

Ocrevus Zunovo can be administrated by providers twice a year with a 10-minute subcutaneous injection. Ocrevus Zunovo will be available in a few weeks and be priced at parity with Ocrevus IV.

Tecentriq Hybreza will be available in a few weeks and be priced at parity with the IV Tecentriq 1200 mg vial.
Zymfentra is a subcutaneous infliximab product used to treat ulcerative colitis and Crohn��’s disease. It has a price of $6,181.08 for two shots over four weeks.

The FDA has given mirdametinib a Prescription Drug User Fee Act (PDUFA) action date of Feb. 28, 2025, to treat patients with neurofibromatosis type 1 with tumors that grow along the peripheral nerve.

The FDA has assigned a Prescription Drug User Fee Act of Dec. 20, 2024, for Zynquista (sotagliflozin) to control glycemic levels in adults with type 1 diabetes and chronic kidney disease.
The FDA identified at a third party fill and finish manufacturer. The issues have been resolved, and reinspection is expected in the next few months.
Livdelz is an oral therapy to treat patients with primary biliary cholangitis, a progressive autoimmune disease. It is expected to be available in pharmacies next week.

Zymfentra launched in March 2024 as the first subcutaneous formulation of infliximab for patients with ulcerative colitis and Crohn’s disease. It has a list price of $6,181.08 for two shots over four weeks.

Zunveyl, a prodrug of galantamine that addresses the gastrointestinal side effects, will be available in the first quarter of 2025.

Leqselvi is approved to treat adults with severe alopecia. One-third of patients in clinical trials experienced 80% scalp hair coverage at 24 weeks.

Duvyzat was approved in March 2024 and works to reduce the inflammation and loss of muscle experienced by patients with Duchenne muscular dystrophy.
Epysqli is a monoclonal antibody that is approved to treat two rare conditions that break down red blood cells.
Boehringer Ingelheim’s high- and low-concentration adalimumab-adbm will be available through GoodRx for $550 for two-pack, which represents a 92% discount from the Humira list price.
If approved, tabelecleucel would be the first therapy specifically to treat Epstein-Barr virus related post-transplant lymphoproliferative disease. The FDA’s action date is Jan. 15, 2025.

OX124 is a nasal spray provides rapid absorption of naloxone for patients experiencing an opioid overdose. The FDA would like to see additional technical data, as well as data on whether patients can correctly use the device.
Giant cell arteritis is an inflammatory disease that affects the large blood vessels that supply blood to the head and brain.
Genentech had recalled the Susvimo ocular implant two years ago. The FDA has approved changes to the implant and needle.
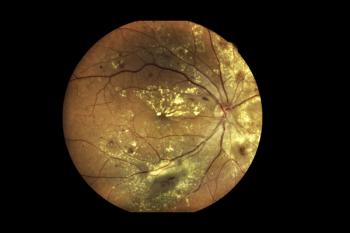
The prefilled syringe of Vabysmo will become available in the coming months. It treats age-related macular degeneration, diabetic macular edema and macular edema following retinal vein occlusion.

Samsung Bioepis’ Pyzchiva (ustekinumab-ttwe) will be available beginning Feb. 22, 2025 and will be marketed by Sandoz.
For the second time, the FDA is asking for additional information about chemistry manufacturing and controls.
Epkinly is a bispecific antibody now approved to treat both relapsed or refractory follicular lymphoma and relapsed or refractory diffuse large B-cell lymphoma.

The FDA cited issues related to a third-party manufacturer. The agency did not request any additional testing from AbbVie.

The recalled batches have been found to have potassium chloride that does not dissolve, which can cause high potassium levels that can lead to hypertension, heart failure, or renal dysfunction.
Krazati is already available to treat patients with KRAS G12C-mutated locally advanced or metastatic non-small cell lung cancer.

Skyrizi is also available to treat patients with plaque psoriasis, psoriatic arthritis, and Crohn’s disease and has a list price of $21,017.36 for one dose.
Tagrisso, which is already available to treat patients with non-small cell lung cancer and EGFR mutations, is being reviewed by the FDA for patients with this cancer and exon 19 deletions or exon 21 mutations.
The FDA is considering a monthly IV maintenance dose of Leqembi to treat people with Alzheimer’s disease. The action date is set for Jan. 25, 2025.

Vanity Fair shines a light on how fake Ozempic and other semaglutide products have found their way into the U.S. supply chain.