Non-invasive Test That May Help Predict Eczema in Babies
Results from the test reveal that high levels of the immune biomarker Thymus and Activation-Regulated Chemokine (TARC) in babies increases their chances of developing atopic dermatitis.
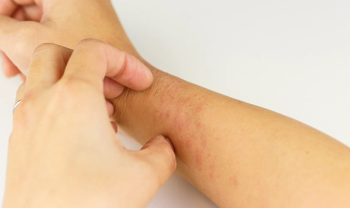
There is a non-invasive test that may help predict eczema development in babies, according to a study published in BMJ Open.
This test was conducted by researchers at the University of Copenhagen in Denmark. The goal was to identify early predictors of atopic dermatitis during the first 2 years of life. The study also assessed patient and parental characteristics, family history of atopic comorbidities, exposures during pregnancy and in infancy, and skin barrier function and development.
Results of the study recently shared in September at the 31st European Academy of Dermatology and Venereology (EADV) Congress, included 450 children - 300 being term newborns and 150 preterm newborns.
In the study, researchers used tape strips to collect skin samples on the newborns to test for skin biomarkers. In term children, skin samples were collected from the cheek and back of the hand within the first few days of life and again at 2 months. In preterm children, samples were from between the shoulder blades within the first month of life and also from the cheek at 2 months.
It was reported the immune biomarker, Thymus and Activation-Regulated Chemokine (TARC), was a key predictor of atopic dermatitis. If TARC levels were high in babies, they had a better chance of developing atopic dermatitis.
At the two-month marker, elevated TARC levels were associated with the onset of atopic dermatitis before 24 months, particularly among term participants. Elevated TARC levels with preterm babies had a significant association with atopic dermatitis. Among term infants, elevated levels of the biomarkers interleukin 8 and interleukin 18 were associated with developing moderate to severe atopic dermatitis.
Eczema can affect people of all ages and can appear in many forms. Early identification of this form of eczema can aid in effective treatment.
Potential limitations of the BABY Cohort is that all term children are recruited from Copenhagen only, possibly limiting the generalisability of the study to more rural areas. As the study is strictly non-invasive, blood measurements were not made either, therefore not assess the possible role of systemic inflammation.
Newsletter
Get the latest industry news, event updates, and more from Managed healthcare Executive.