
The agency cited an open inspection issue at a facility that performs analytical testing of drug substance.


The agency cited an open inspection issue at a facility that performs analytical testing of drug substance.

In a study conducted by JAMA, results were recently released sharing children with active asthma had reduced symptom days, but not significantly, in the use of a school-wide integrated pest management program or a high-efficiency particulate air filter purifier in recorded 2-week time periods.

Efforts are underway to accelerate the development of a novel therapy for excessive airway mucus and inflammation treatment for patients with chronic obstructive pulmonary disease and non-cystic fibrosis bronchiectasis.

Three experts say that pulmonary rehabilitation programs don’t necessarily result in improved lung function but are associated with improved physical and other sorts of functioning. Less than 5% of those who might benefit from pulmonary rehabilitation services received them.

Tezepelumab, which is being currently reviewed by the FDA, provides small improvements in daily symptoms and quality of life but is only modestly cost-effectiveness at the anticipated price, according to the cost-effectiveness group's calculations.

Investigators found patients with COPD taking Anoro Ellipta had low overall on-treatment exacerbation rates, which can reduce medical costs due to increased adherence to therapy.

The agency also granted tezepelumab priority review with a PDUFA date sometime in the first quarter of 2022.

Regulatory actions for drugs for children, a combination cancer therapy, launches of two generic therapies for COPD, and cancer and Alzheimer’s therapies receive Breakthrough Designation round out this week’s FDA news.

With more than 50 million Americans suffering from allergies each year, it’s critical for primary care physicians to play a pivotal role in appropriately managing asthma and allergic diseases.

In this week's episode of Tuning In to the C-Suite podcast, MHE's Briana Contreras spoke with Dr. Rob Kowal, chief medical officer of the Cardiac Rhythm and Heart Failure division at Medtronic. The two discussed how remote monitoring and IoT is changing healthcare and how remote technology is also gaining a wide-spread adoption to monitor patients at home who have chronic conditions like heart failure, chronic obstructive pulmonary disease and diabetes.

The COPD Foundation is calling for a change in clinical trial design that would test experimental therapies in subsets of patients.
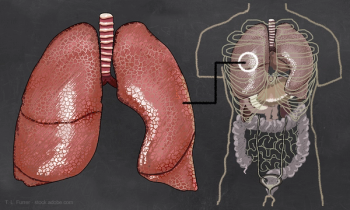
The first respiratory biologic to be approved for administration via an autoinjector will be available in the US soon.
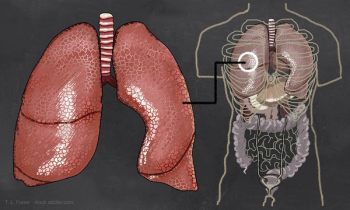
FDA approved the first generic of a popular inhaler to treat asthma and chronic obstructive pulmonary disease

Two recent studies demonstrate that pirfenidone (Esbriet, Genentech) prolongs life expectancy by 2.47 years for IPF patients and that Esbriet and nintedanib (Ofev, Boehringer Ingelheim) reduce the decline in lung function.

Pete Mulliner, an IPF patient and ambassador for the Pulmonary Fibrosis Foundation, discusses how he believes physicians should treat patients who have been recently diagnosed with IPF.

Boehringer Ingelheim recently began a clinical trial to assess the safety and tolerability of adding on pirfenidone to Ofev (nintedanib) for idiopathic pulmonary fibrosis patients.

The Pulmonary Fibrosis Foundation and Boehringer Ingelheim’s new five-year partnership, aims to raise awareness of pulmonary fibrosis, provide disease education and advance care and funding research for the PF community

Most idiopathic pulmonary fibrosis patients have considerably impaired lung function and gastroesophageal reflux disease is the most common co-morbidity, according to initial results from the IPF-PRO Registry.

A new survey of over 400 pulmonologists from 10 countries found that 88% of pulmonologists believe a delay in the diagnosis of idiopathic pulmonary fibrosis negatively impacts patients.

Janet Woodcock, MD, director of the Center for Drug Evaluation and Research at the FDA, will speak about the future of drug development in pulmonary fibrosis during the PFF Summit 2015: From Bench to Bedside conference, November 12-14 in Washington D.C.

FDA approved the dual combination bronchodilator Utibron Neohaler (indacaterol/glycopyrrolate) and the stand-alone monotherapy Seebri Neohaler (glycopyrrolate) – which is one component of Utibron Neohaler -- for patients with chronic obstructive pulmonary disease (COPD), including chronic bronchitis and/or emphysema.

When two new treatments for idiopathic pulmonary fibrosis (IPF) were approved by FDA last year, there was not much data available on the drugs in patient populations.

Here are 3 major concerns patients with IPF should be aware of, according to research from Carlo Vancheri that appeared in a recent issue of Sarcoidosis Vasculitis and Diffuse Lung Diseases.

Pulmonary fibrosis (PF) organizations and healthcare providers are lauding the update of the Official ATS/ERS/JRS/ALAT Clinical Practice Guideline: Treatment of Idiopathic Pulmonary Fibrosis, which was last updated in 2011.

Healthcare providers, celebrities, patients and others are helping to increase awareness of idiopathic pulmonary fibrosis (IPF) during the Pulmonary Fibrosis Foundation’s (PFF) Pulmonary Fibrosis Awareness Month.